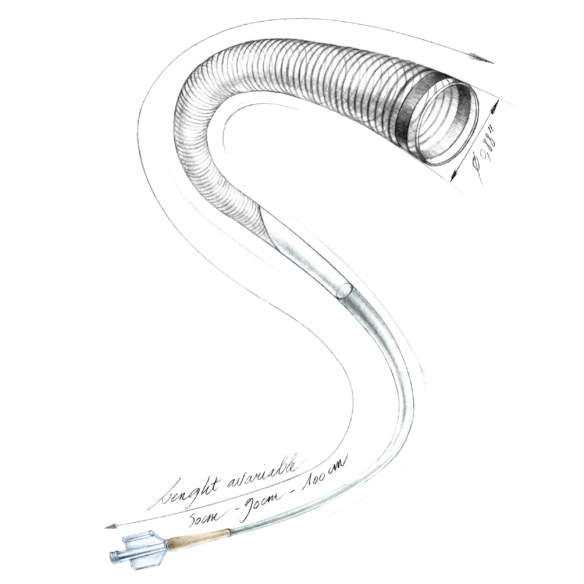
Key Differentiators
- DMSO compatible microcatheter
- 4 transition zones to support navigability
Legal Mentions
Gama+, reinforced microcatheters intended for use in interventional radiology procedures in the vascular and neurovascular system for: the injection of diagnostic or therapeutic products, the insertion of compatible pushable coils or detachable coils, the insertion of compatible intracranial self-expandable stents, the insertion of compatible thrombo-embolectomy devices. Class III CE0297 in compliance with Medical Device Directive (MDD 93/42/EEC amended by 2007/47/EC). Manufactured by BALT EXTRUSION SAS. Carefully read the instructions for use before use. First CE marking: 2019.
Indication for Use
Not Cleared or Approved For Commercial Use in USA

Interested in learning more about this product?
Gama+ | Reinforced Microcatheter